Canadian researchers from McGill University have recently found new and affordable materials that could prove key for improving the batteries used in electric vehicles. The breakthrough was analysed and confirmed with the Canadian Light Source (CLS) at the University of Saskatchewan.
Read next: Hyundai, Kia and LG Chem launch EV and battery challenge for startups
The research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) and supported by Hydro-Quebec – a public utility company that manages the generation, transmission and distribution of electricity in the Canadian province of Quebec.
This comes after the move toward greener technologies, increases the need for affordable, safe and powerful batteries. Battery-powered electric vehicles, for example, have much higher safety standards than smartphones, and to travel the long distances required in Canada – a country that undergoes extreme conditions than a lot of places in Europe – the need for lightweight, high-energy capacity batteries can make a world of difference.

Buy a car phone mount on Amazon (Affiliate)
Current rechargeable batteries tend to use expensive non-abundant metals, like cobalt, that carry an environmental and human rights toll under the poor labour conditions in mines in Africa. These create barriers to wider adoption, expansion of the market and manufacturers switching to a fully electric portfolio.
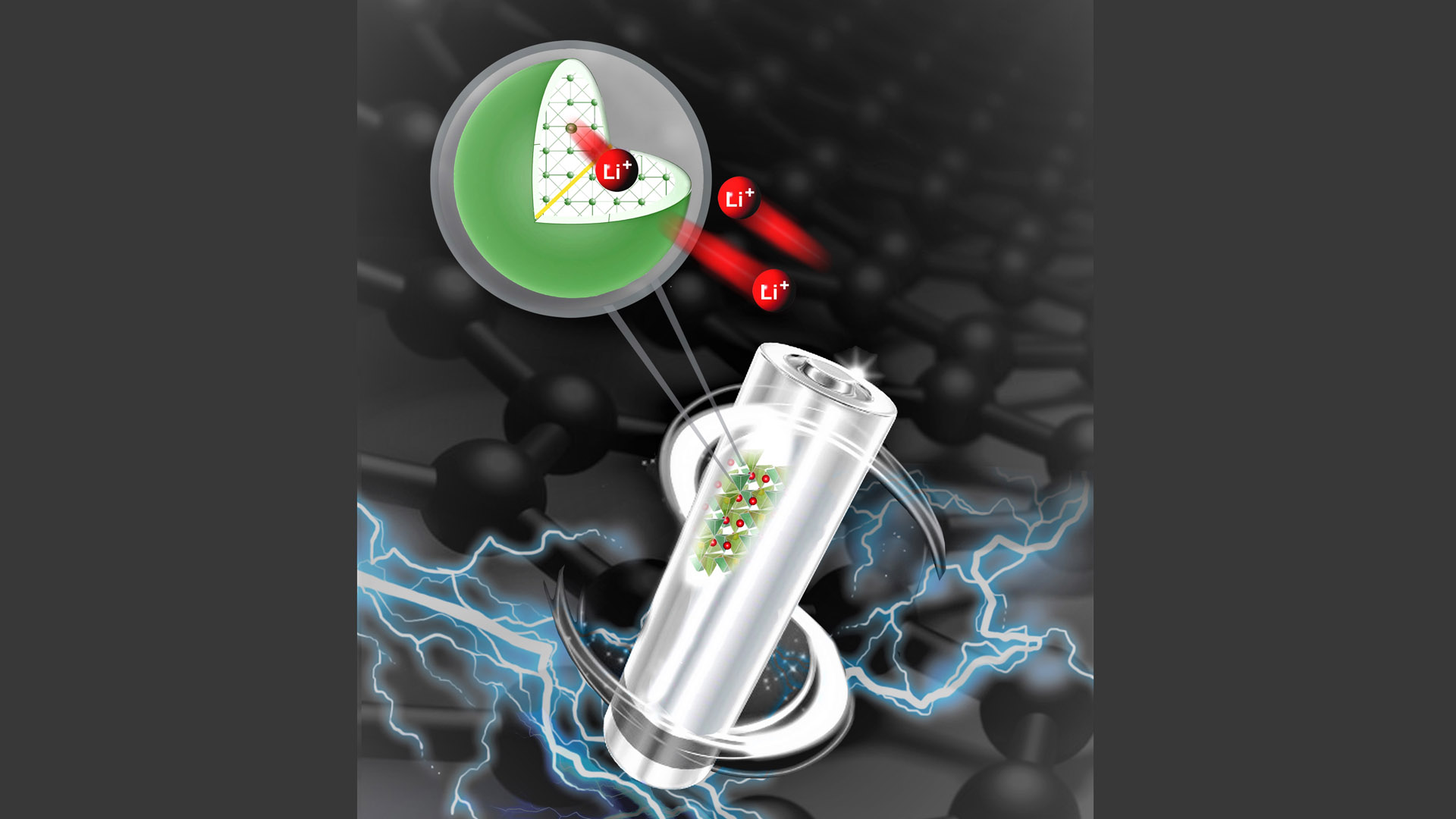
The battery’s cathode, or positive electrode, is one of the best candidates for Li-ion battery improvement. “Cathodes represent 40% of the cost of the batteries that we are using in our phones right now. They are absolutely crucial to improve battery performance and reduce dependency on cobalt,” says Majid Rasool, lead author on the paper published in Advanced Materials Interfaces. Rasool completed his PhD at McGill while studying battery cathode materials.
A McGill team’s research focuses on a promising battery cathode made of iron and silicon, which are abundant and inexpensive materials. This particular type of cathode, lithium iron silicate, has theoretically a high capacity to hold energy over several charge-discharge cycles, but in practice, this had not been achieved.
The group’s breakthrough comes thanks to applying an electronically conducting polymer called Poly(3,4-ethylenedioxythiophene) or also known as ‘PEDOT’ on the surface of cathode nanocrystals through a controlled interfacial reaction. Testing revealed an impressive initial battery capacity as well as improved performance over many charging cycles.
Read next: This new UK test facility will streamline EV battery development
“We were not expecting that big of a jump in performance over carbon coating. I knew this could work, but when we got the results, it was really quite fascinating. We chose to work with this particular material in close collaboration with Hydro-Quebec because it’s sustainable, low cost and safe”
Using the soft X-ray Spectromicroscopy (SM) and the Canadian Macromolecular Crystallography Facility (CMCF) at the CLS, it was possible to explain the impressive jump in performance that the coated nanocrystal approach enabled.
“The CLS was very helpful to let us dig deeper and begin to explain why the PEDOT coating treatment and the sub-surface iron-rich layer improved performance so much.”
Read next: Recyclability of vehicles: What impact does it have on our environment?
Encapsulating nanocrystals in PEDOT was technically difficult to achieve, taking nearly two years of effort, but resulted in ‘remarkable enhancements’ to performance, both in terms of initial and long-term capacity. “There’s still work to be done to understand why and build on this, but this coating process opens up new engineering strategies for batteries,” says Rasool.





